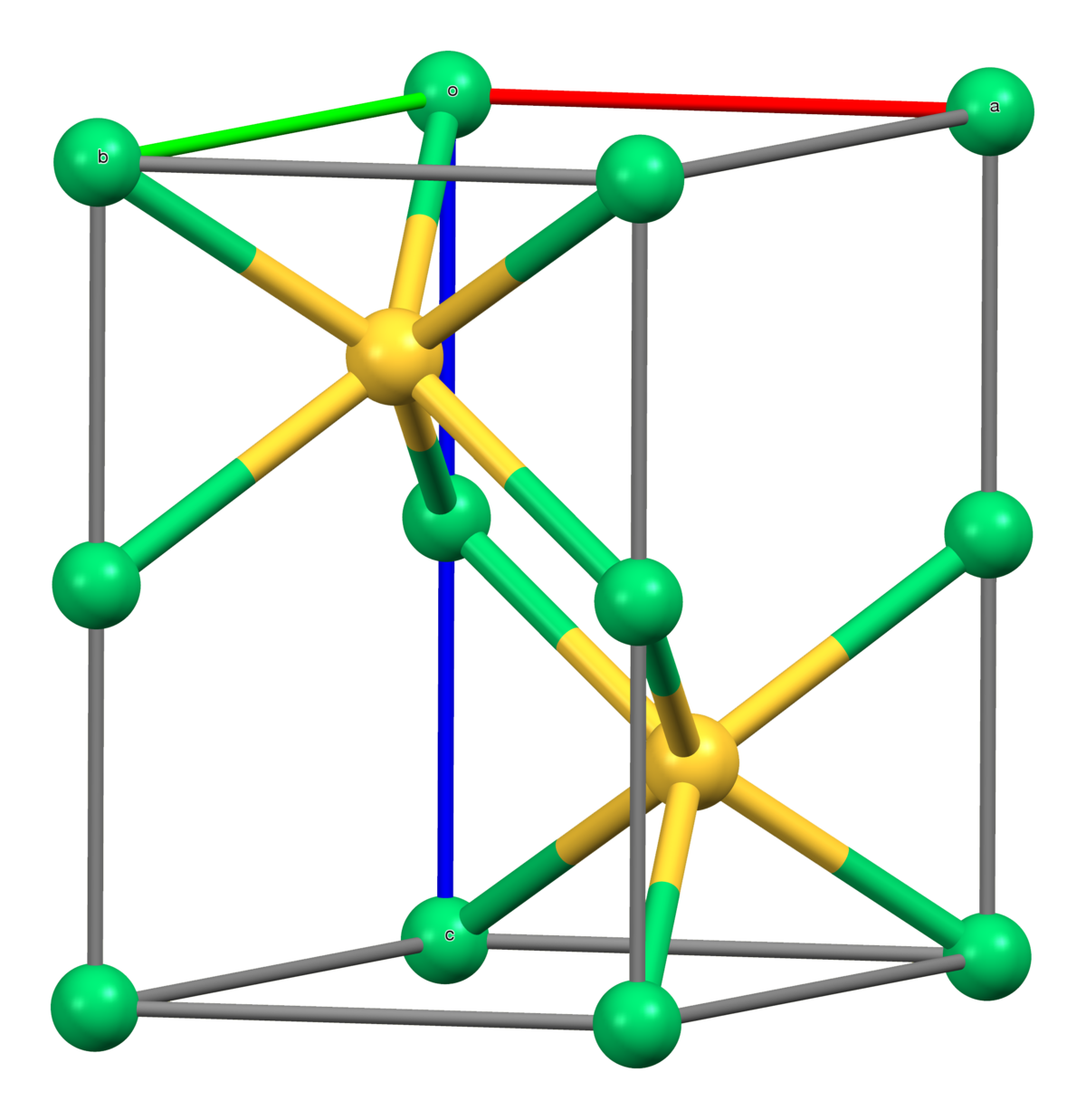
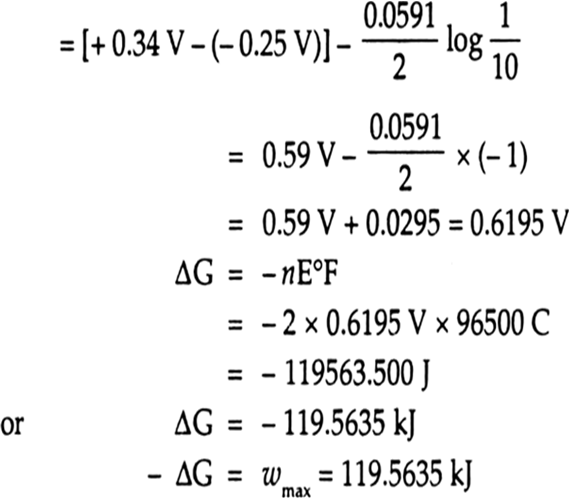Three-Dimensional Interconnected Binder-Free Mn–Ni–S Nanosheets for High Performance Asymmetric Supercapacitor Devices with Exceptional Cyclic Stability | ACS Applied Energy Materials

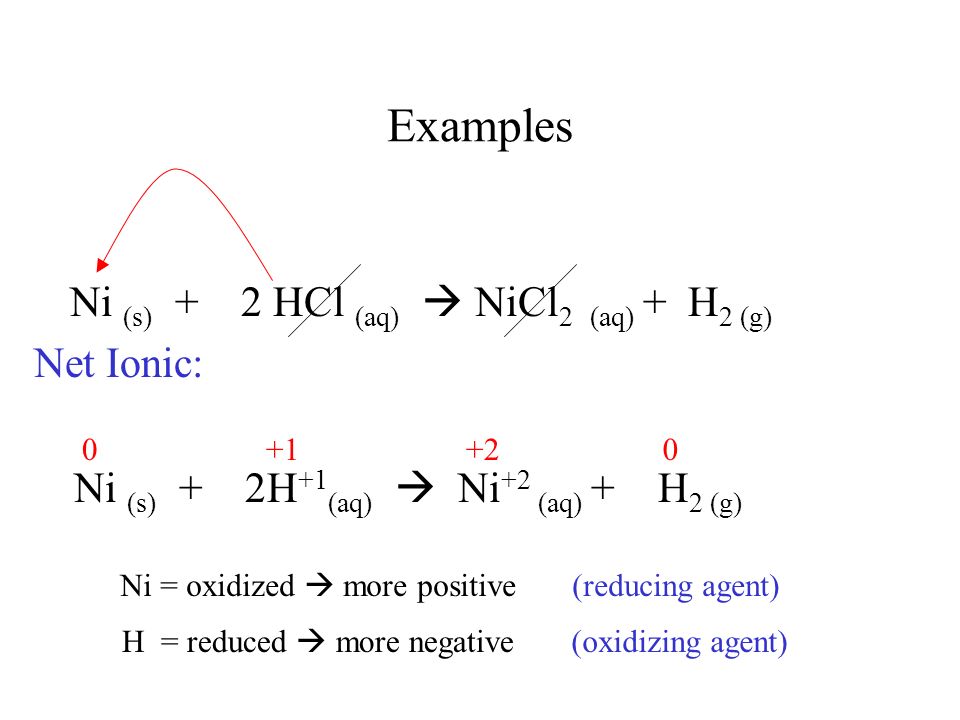
One-Step Synthesis of Nickel Sulfides and Their Electrocatalytic Activities for Hydrogen Evolution Reaction: A Case Study of Crystalline h-NiS and o-Ni9S8 Nanoparticles | ACS Applied Energy Materials

Surface Restructuring of Nickel Sulfide Generates Optimally Coordinated Active Sites for Oxygen Reduction Catalysis - ScienceDirect

Surface Activation and Ni‐S Stabilization in NiO/NiS2 for Efficient Oxygen Evolution Reaction - Zhang - 2022 - Angewandte Chemie International Edition - Wiley Online Library

Interface Engineering of NixSy@MnOxHy Nanorods to Efficiently Enhance Overall-Water-Splitting Activity and Stability | Nano-Micro Letters

Calculate the emf of the cell in which the following reaction takes place: Ni(s)+2Ag^+(0.002M)→ Ni^2+(0.160 M)+2Ag(s) Given that E^∘_cell=1.05 V nbsp;

Heterogeneous histories of Ni‐bearing pyrrhotite and pentlandite grains in the CI chondrites Orgueil and Alais - Berger - 2016 - Meteoritics & Planetary Science - Wiley Online Library

A porous proton-relaying metal-organic framework material that accelerates electrochemical hydrogen evolution | Nature Communications

Engineering Sulfur Vacancies of Ni3S2 Nanosheets as a Binder-Free Cathode for an Aqueous Rechargeable Ni-Zn Battery | ACS Applied Energy Materials

4 15. E.M.F. of Ni(s)[Ni2+ (aq) || Cu2+ (aq)|Cu(s) cell can be increased by (1) Adding NH, in the right half-cell (2) Increasing the conc. of Ni2+ ions (3) Adding dimethyl

science chemistry precipitation reaction nickel hydrochloric acid | Fundamental Photographs - The Art of Science
![The Nernst equation the following electrochemical cell will be: Ni(s) | Ni2+ (aq)|| Ag+ (aq)| Ag A) Ecell = Eºcell-RT/F[In[Ni2+]/[Ag+12] B) Ecell = Eccl1-RT/2F[In[Ni2+1/[Ag+1?] C) Ecell = Eºcell-RT/2F[In[Ag+]2/[Ni2+]] D) Ece = Eccl1-RT/2F[In[Ni2+1/[Ag+l] The Nernst equation the following electrochemical cell will be: Ni(s) | Ni2+ (aq)|| Ag+ (aq)| Ag A) Ecell = Eºcell-RT/F[In[Ni2+]/[Ag+12] B) Ecell = Eccl1-RT/2F[In[Ni2+1/[Ag+1?] C) Ecell = Eºcell-RT/2F[In[Ag+]2/[Ni2+]] D) Ece = Eccl1-RT/2F[In[Ni2+1/[Ag+l]](https://toppr-doubts-media.s3.amazonaws.com/images/2126244/274414aa-deba-45ea-a8ef-39b8460abe5f.jpg)
The Nernst equation the following electrochemical cell will be: Ni(s) | Ni2+ (aq)|| Ag+ (aq)| Ag A) Ecell = Eºcell-RT/F[In[Ni2+]/[Ag+12] B) Ecell = Eccl1-RT/2F[In[Ni2+1/[Ag+1?] C) Ecell = Eºcell-RT/2F[In[Ag+]2/[Ni2+]] D) Ece = Eccl1-RT/2F[In[Ni2+1/[Ag+l]